Dear support, thanks for your continued help.
My queries concern the following survey IDs 4539 and 4538. They are the same except for different groups and therefore 4538 has a few items less. We made two projects with separate surveys to keep our two groups separate (otherwise there would be one registration code). We did this also because we wanted to change the name of the project (which is displayed in the informed consent), but could not (so we made a new project).
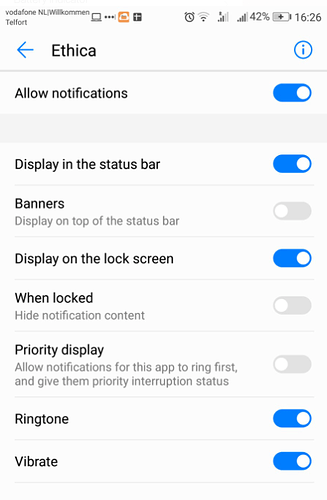
Notification/push notification
We pretested the surveys in the new app, but we do not get any notification that prompts us to open the app. We have assigned notification rights to the app (they were there from the start and seem correct).
We were wondering whether this has something to do with the new app?
New app/iOS
When pretesting the iOS version, we found that the informed consent was not scrollable (we searched for the usually hidden scroll bar but didn’t see it).
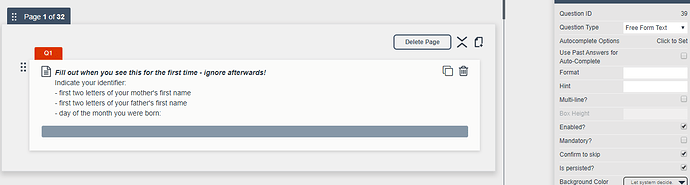
Filling out an item once during the ten days
There are two items that participants only need to fill out once - assuming that we will have data from each participant in identifiable. I was wondering whether I could make the items ‘persistent’ but when filling out the survey again, they show up again (so persistence seems to be only relevant for one survey/within one survey). Is there a way to have participants only fill out these identifier items when they fill out their first survey, but not afterwards?
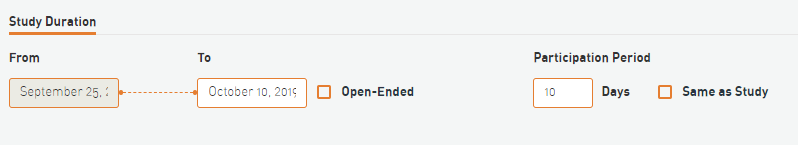
Starting point
In Survey 4538, I am unable to edit the starting point of the project (to October 1st). Please see the screenshot below:
Thank you very much for your help!